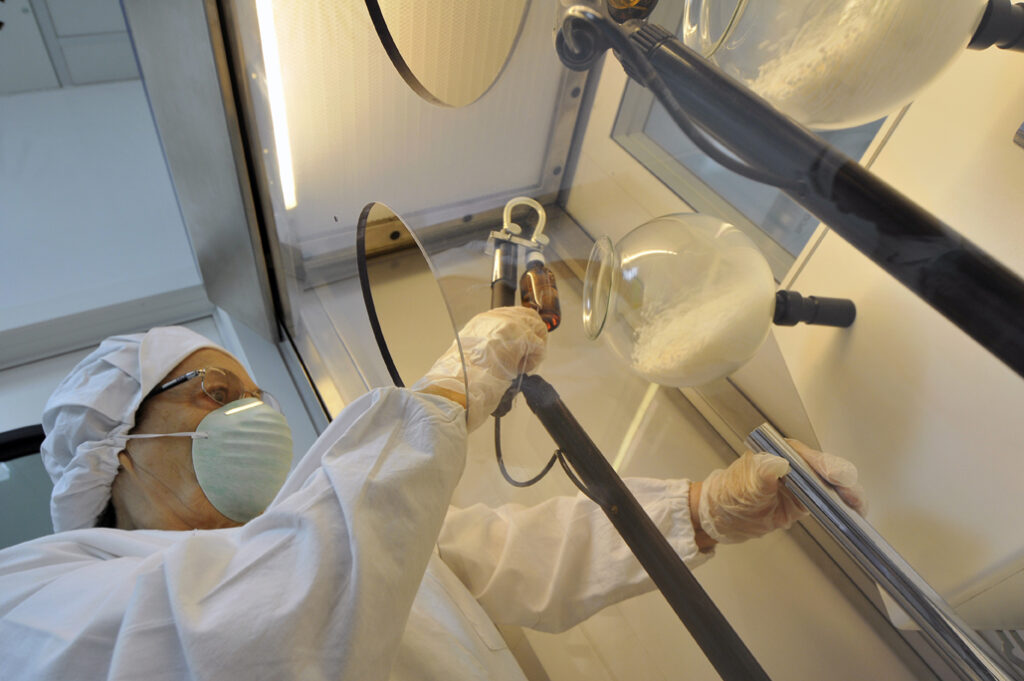
We are one of the few Italian company that produces mother tinctures and glycerin macerated starting from the raw material (nosodes, plants, organs, chemicals, animals, venoms)
- Liquids for internal use
- Capsules with impregnated microgranules
- Tubes and single-dose tubes with impregnated microgranules
- Single-dose vials
- Raw materials of plant origin (mother tinctures, glycerinated macerates) and organic origin (nosodes and organs)
- Consultancy concerning relevant national regulations
- Drafting of common technical documents (CTD)
- Management of the procedures concerning CTDs and regulation files to be provided to AIFA (Italian Medicines Agency) in order to obtain the AIC product placement authorization
- Direct contact with databases
- Physicochemical testing
- Microbiological testing
- In compliance with the ICH guidelines